

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50012070 (5-Hydroxy-2-methyl-[1,4]naphthoquinone | 5-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM81348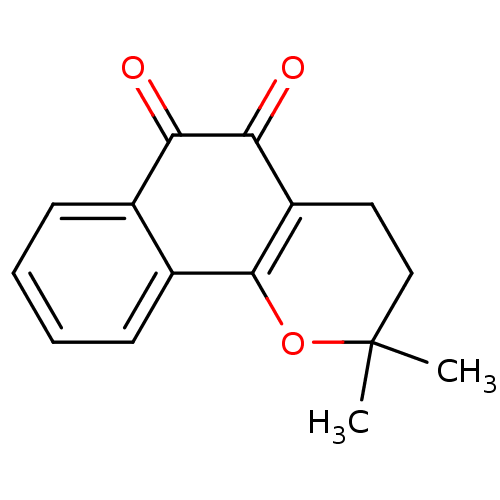 (β-Lapachone (A3) | Beta lapachone | R115 (Rea...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM60918 (5-Amino-2-hydroxy-benzoic acid | MESALAMINE | MLS0...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM26655 (2-(3,4-dihydroxyphenyl)-3,5,7,8-tetrahydroxy-4H-ch...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50523758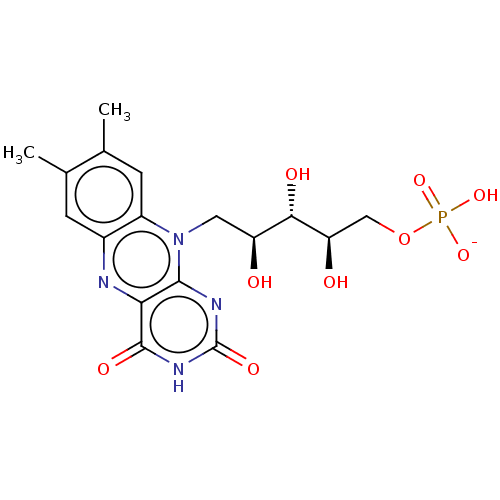 (Phosphated riboflavin | Photrexa | RIBOFLAVIN 5'-P...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50105463 (2,7-diamino-10-ethyl-9-phenylphenanthridinium brom...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM24778 (2-methyl-1,4-dihydronaphthalene-1,4-dione | 2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM67454 (1,2,4-trihydroxy-9,10-anthraquinone | 1,2,4-trihyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM71542 ((4-amino-1-methyl-butyl)-(6-methoxy-8-quinolyl)ami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50434369 (METHYLENE BLUE CHLORIDE HYDRATE | Methylene Blue |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50070942 ((-)-Epigallocatechin gallate | (-)-Epigallocatechi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50523760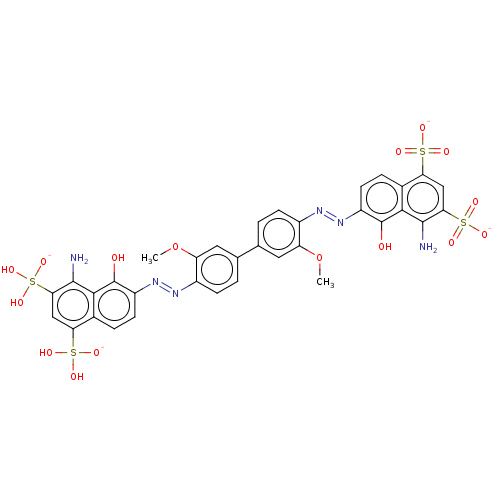 (CHEMBL4448756) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50366944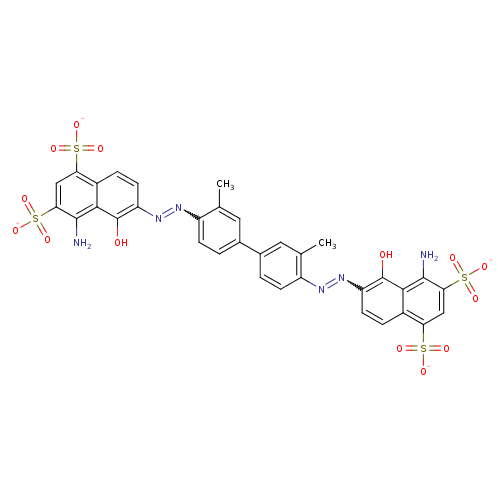 (Azovan Blue | Azovan sodium | EVANS BLUE) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50362895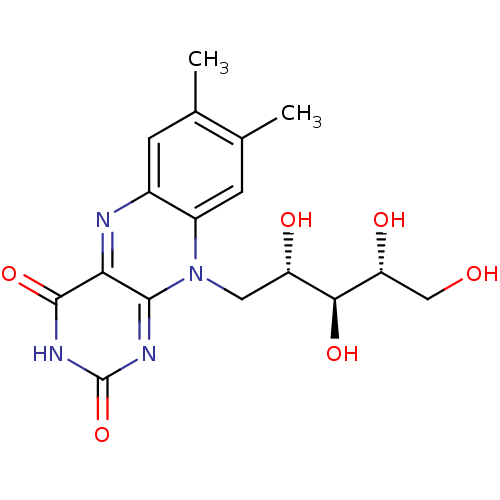 (Lactoflavin | RIBOFLAVIN) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50523756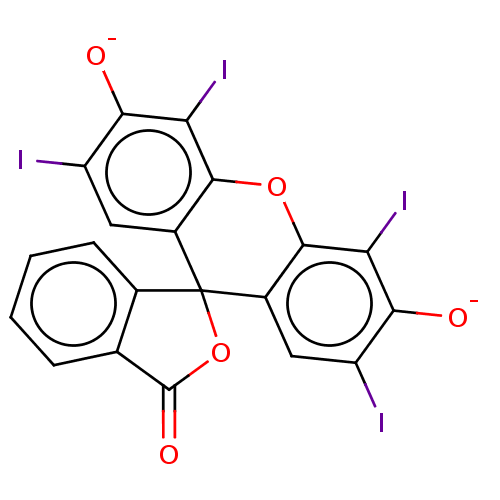 (ERYTHROSINE SODIUM) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50132101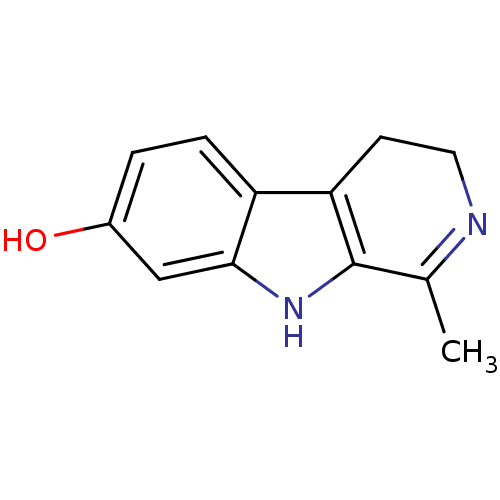 (1-Methyl-4,9-dihydro-3H-beta-carbolin-7-ol | CHEMB...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM23223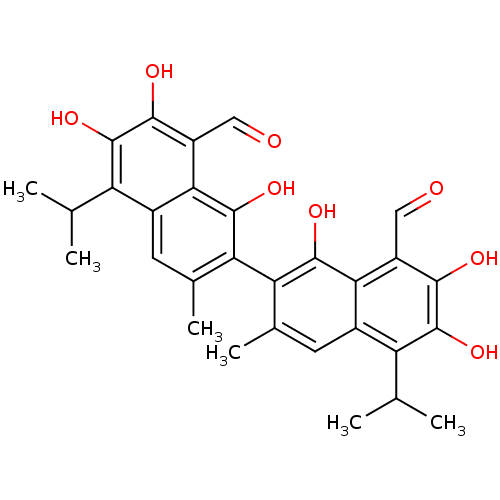 (7-[8-formyl-1,6,7-trihydroxy-3-methyl-5-(propan-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM152157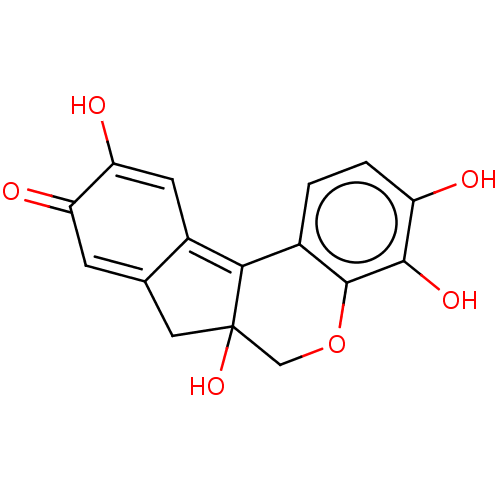 (US8987474, Hematin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50052802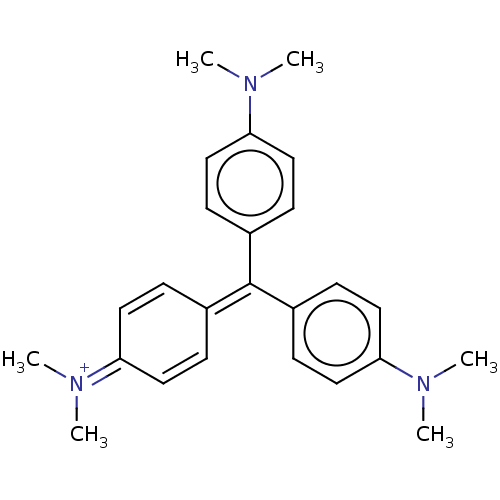 (CHEBI:77181 | CRYSTAL VIOLET | Crystal violet (15)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM60986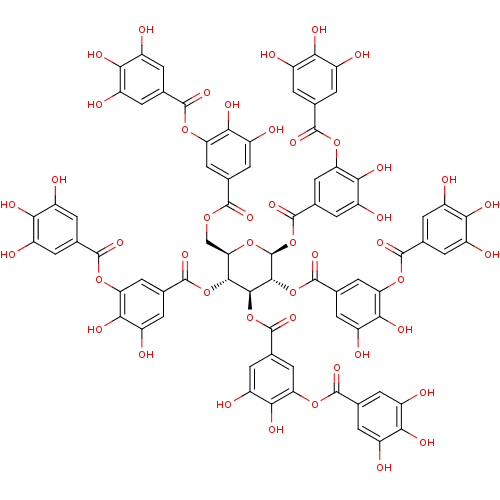 (MLS001335996 | SMR000857330 | TANNIC ACID | cid_16...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM31712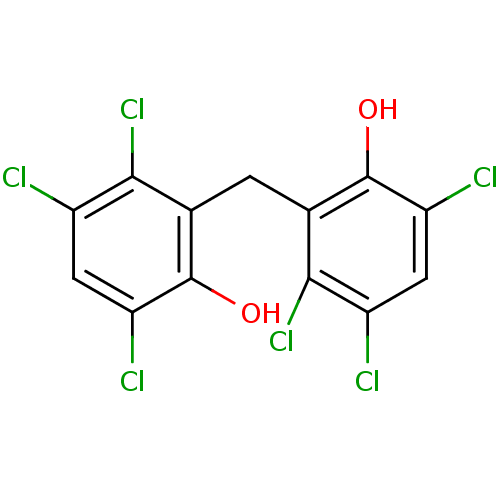 (HEXACHLOROPHENE | Hexach-lorophene | MLS000028433 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50451094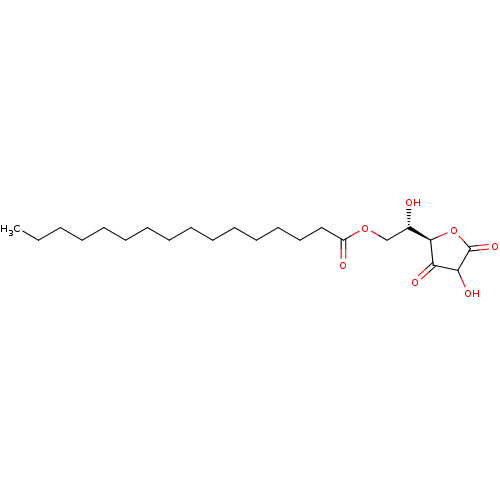 (Ascorbyl Palmitate | Ascorbyl palmitate (E5) | E30...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50450012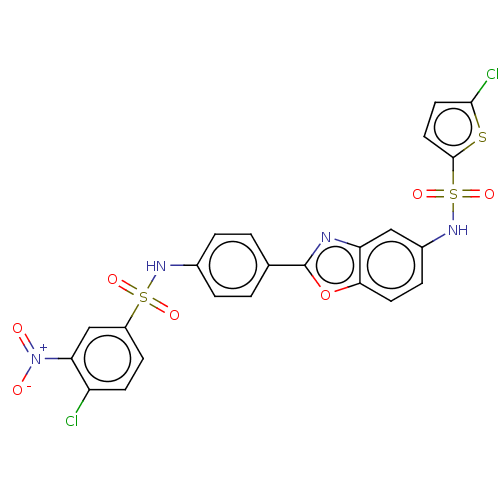 (CHEMBL4167333) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins by measuring MDH enzyme activity using s... | J Med Chem 61: 7345-7357 (2018) Article DOI: 10.1021/acs.jmedchem.8b00989 BindingDB Entry DOI: 10.7270/Q270840G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50140172 (CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM26658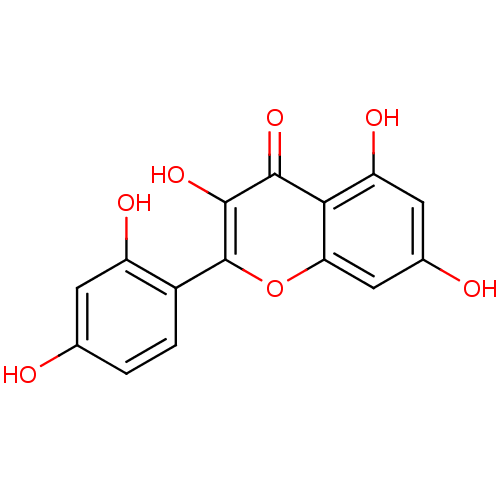 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50410532 (4-NONYLPHENOL) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50450010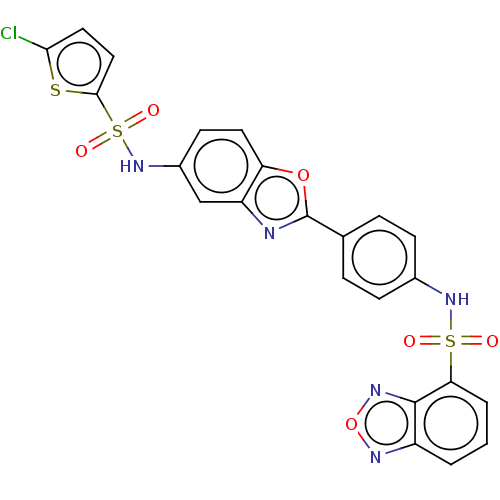 (CHEMBL4168023) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins by measuring MDH enzyme activity using s... | J Med Chem 61: 7345-7357 (2018) Article DOI: 10.1021/acs.jmedchem.8b00989 BindingDB Entry DOI: 10.7270/Q270840G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM31883 (9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50247883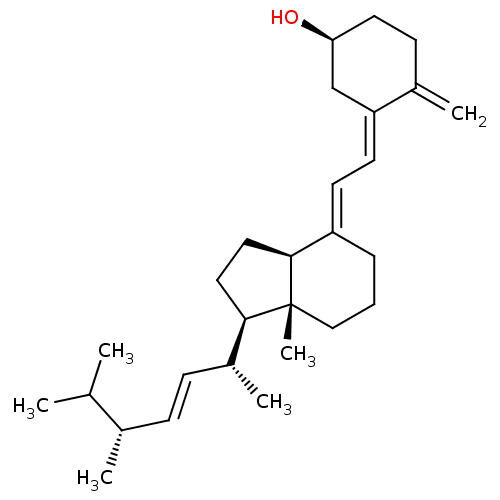 (CHEBI:28934 | Calciferol | Calciferol In Arach Oil...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 BindingDB Entry DOI: 10.7270/Q2NZ8C33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50450007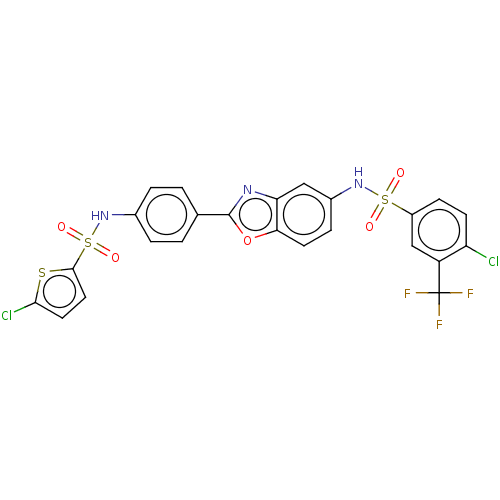 (CHEMBL4169499) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins by measuring MDH enzyme activity using s... | J Med Chem 61: 7345-7357 (2018) Article DOI: 10.1021/acs.jmedchem.8b00989 BindingDB Entry DOI: 10.7270/Q270840G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50450004 (CHEMBL4160098) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins by measuring MDH enzyme activity using s... | J Med Chem 61: 7345-7357 (2018) Article DOI: 10.1021/acs.jmedchem.8b00989 BindingDB Entry DOI: 10.7270/Q270840G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50450005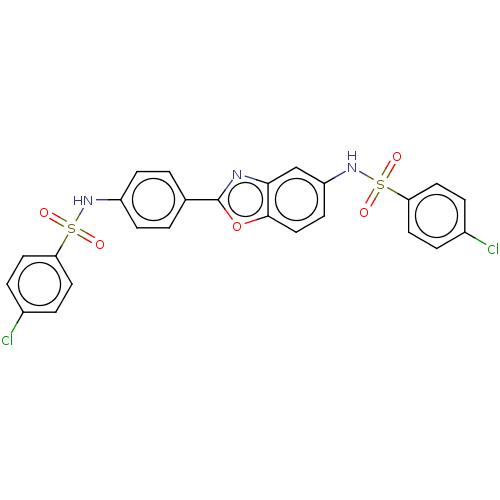 (CHEMBL3975683) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins by measuring MDH enzyme activity using s... | J Med Chem 61: 7345-7357 (2018) Article DOI: 10.1021/acs.jmedchem.8b00989 BindingDB Entry DOI: 10.7270/Q270840G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50450008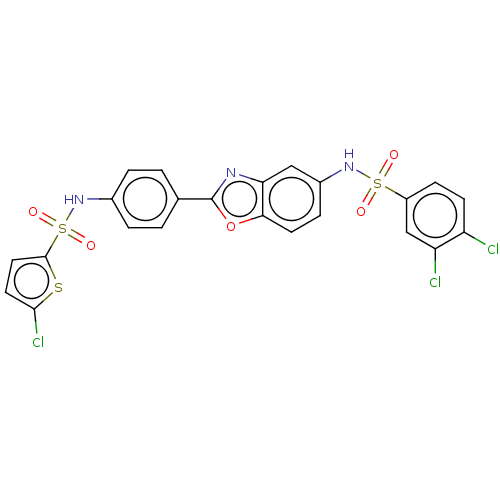 (CHEMBL4166077) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins by measuring MDH enzyme activity using s... | J Med Chem 61: 7345-7357 (2018) Article DOI: 10.1021/acs.jmedchem.8b00989 BindingDB Entry DOI: 10.7270/Q270840G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50450003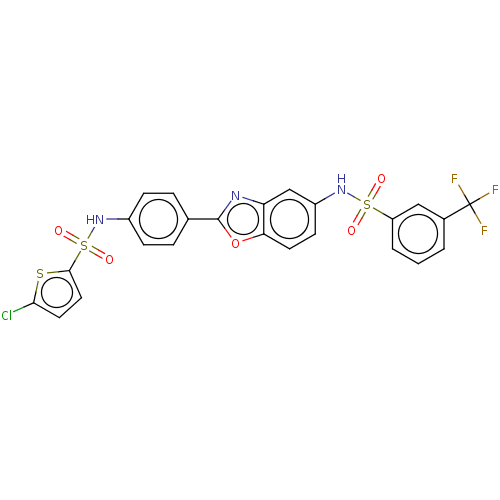 (CHEMBL4176697) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins by measuring MDH enzyme activity using s... | J Med Chem 61: 7345-7357 (2018) Article DOI: 10.1021/acs.jmedchem.8b00989 BindingDB Entry DOI: 10.7270/Q270840G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50450009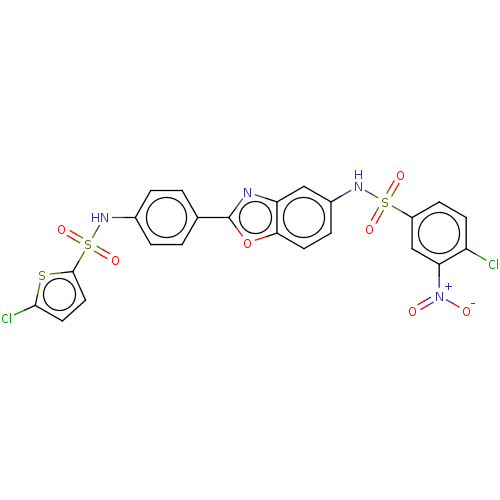 (CHEMBL4161583) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins by measuring MDH enzyme activity using s... | J Med Chem 61: 7345-7357 (2018) Article DOI: 10.1021/acs.jmedchem.8b00989 BindingDB Entry DOI: 10.7270/Q270840G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50450011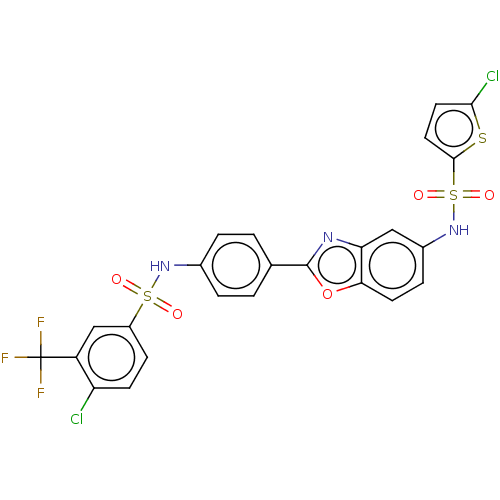 (CHEMBL4161487) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins by measuring MDH enzyme activity using s... | J Med Chem 61: 7345-7357 (2018) Article DOI: 10.1021/acs.jmedchem.8b00989 BindingDB Entry DOI: 10.7270/Q270840G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50450001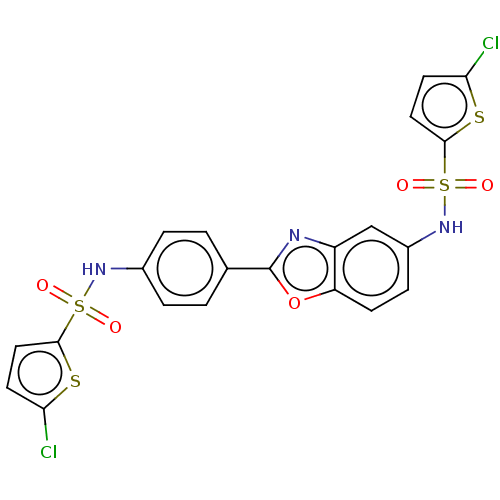 (CHEMBL4162794) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins by measuring MDH enzyme activity using s... | J Med Chem 61: 7345-7357 (2018) Article DOI: 10.1021/acs.jmedchem.8b00989 BindingDB Entry DOI: 10.7270/Q270840G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50450006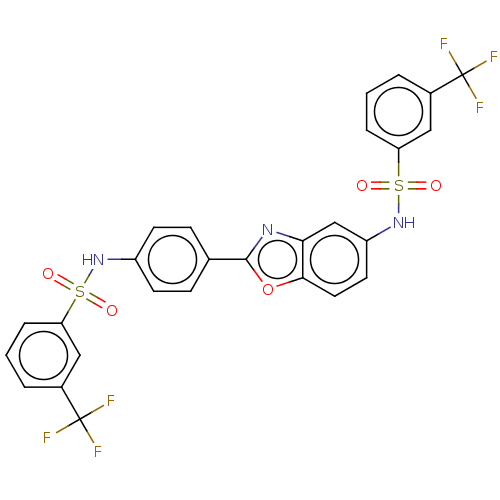 (CHEMBL3970551) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins by measuring MDH enzyme activity using s... | J Med Chem 61: 7345-7357 (2018) Article DOI: 10.1021/acs.jmedchem.8b00989 BindingDB Entry DOI: 10.7270/Q270840G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50450013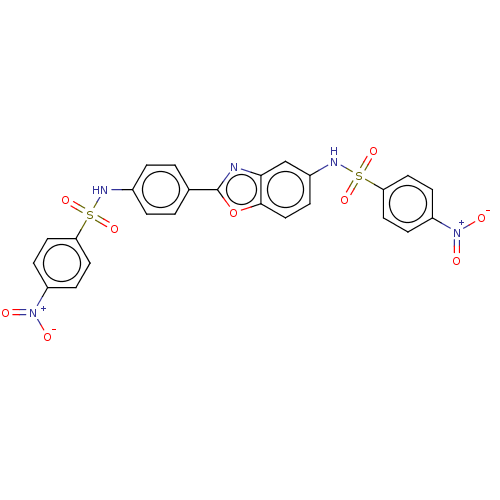 (CHEMBL3955935) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins by measuring MDH enzyme activity using s... | J Med Chem 61: 7345-7357 (2018) Article DOI: 10.1021/acs.jmedchem.8b00989 BindingDB Entry DOI: 10.7270/Q270840G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50468055 (CHEMBL4282354) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins in absence of compound followed by compo... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50335519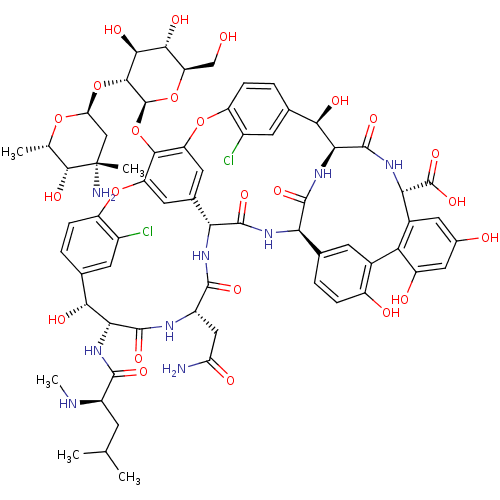 ((S)-3,6-Diamino-hexanoic acid {(3S,9S,12S,15S)-3-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins in absence of compound followed by compo... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50468057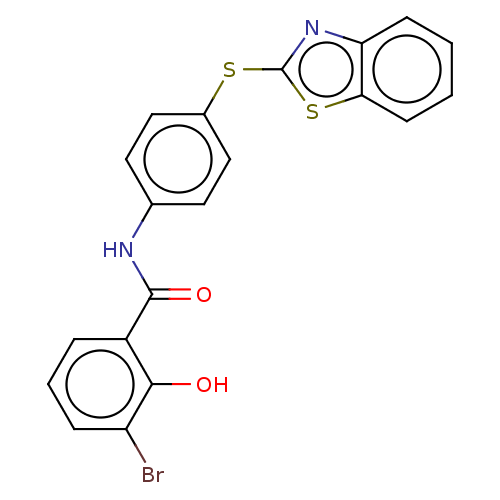 (CHEMBL4281358) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins in absence of compound followed by compo... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM234300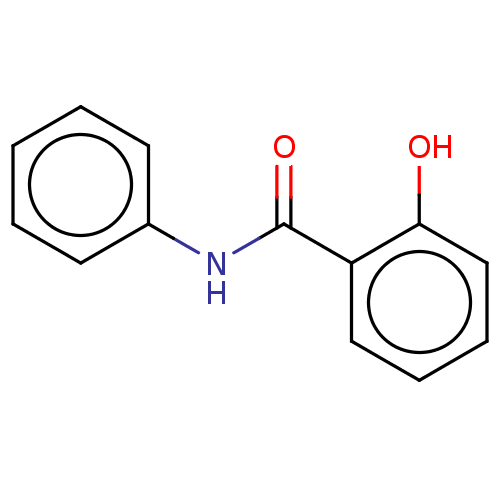 (2-Hydroxy-N-phenylbenzamide (1)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins in absence of compound followed by compo... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50468058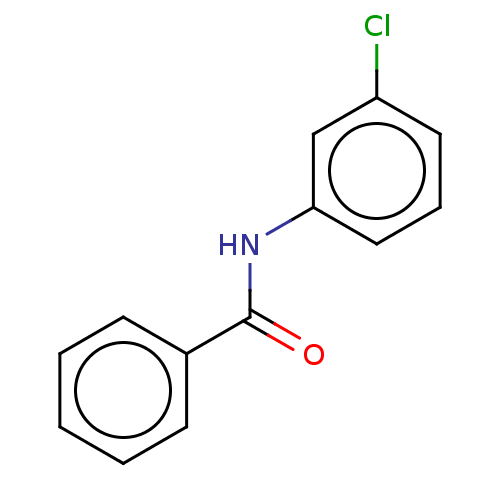 (CHEMBL1559759) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins in absence of compound followed by compo... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50116705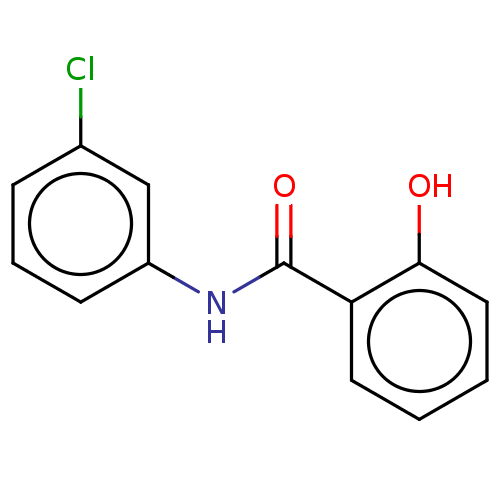 (CHEMBL460481) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins in absence of compound followed by compo... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50468069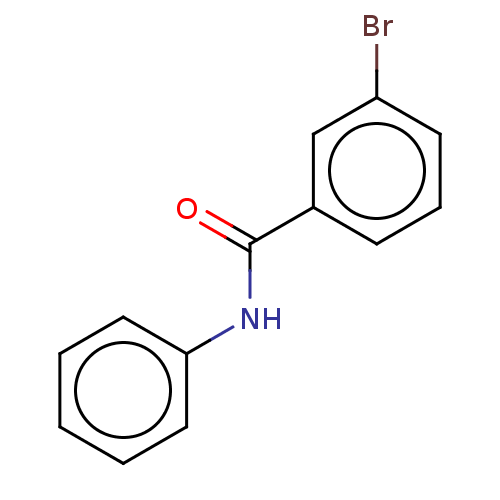 (CHEMBL4292251) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins in absence of compound followed by compo... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50468059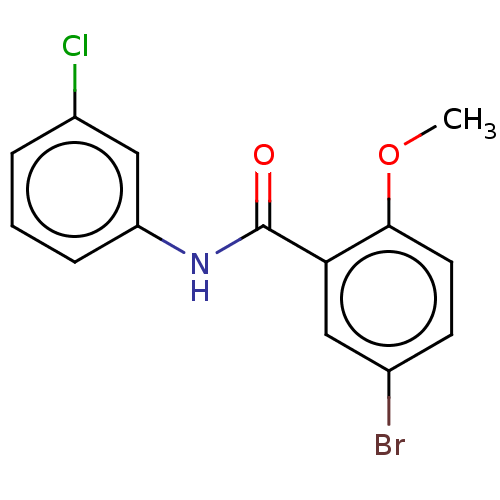 (CHEMBL4284817) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins in absence of compound followed by compo... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50063753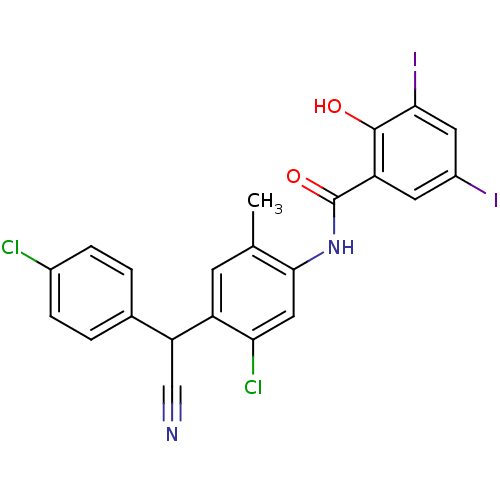 (CHEMBL12131 | Closantel | N-(5-chloro-4-((4-chloro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins in absence of compound followed by compo... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50468074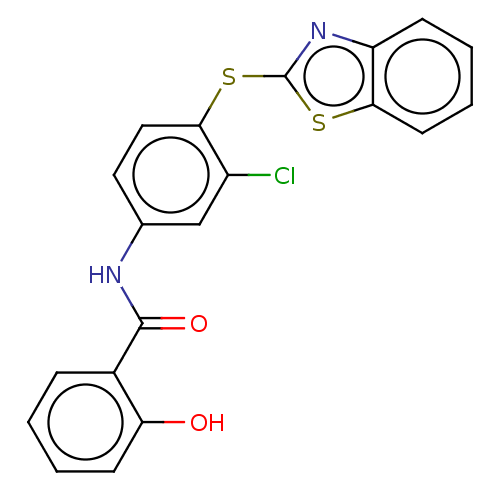 (CHEMBL4284769) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins in absence of compound followed by compo... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50468060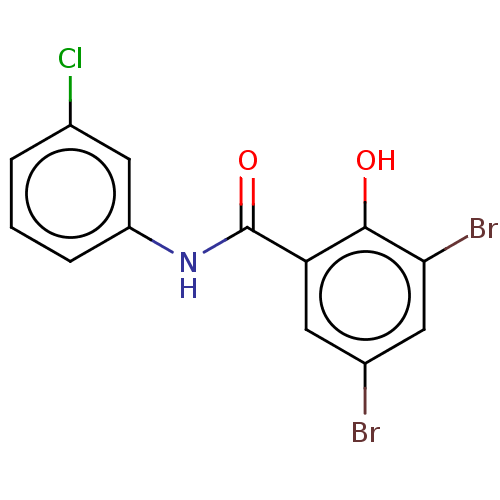 (CHEMBL1631668) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins in absence of compound followed by compo... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 144 total ) | Next | Last >> |